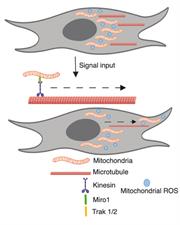
 Mitochondria, the powerhouse of our cells are dynamic organelles that orchestrate numerous cellular process. Mitochondria change shape, size and position within cells and between cells based on cellular cues including metabolic needs, redox status and varying inter- and intracellular stimuli. The movement of mitochondria is coordinated by a specific set of adapter proteins that connect mitochondria to microtubule motors for transport. The transport of mitochondria to specific subcellular locations influences the local metabolic state and induces cell responses. For example, our work has demonstrated that mitochondrial movement to the leading edge of migrating cells fuels cell migration, a process required for tumor cell metastasis. Projects in the lab focus on determining factors that govern subcellular mitochondrial movement and how local mitochondrial metabolites regulate cell signaling pathways and downstream cell outcomes.
Mitochondria, the powerhouse of our cells are dynamic organelles that orchestrate numerous cellular process. Mitochondria change shape, size and position within cells and between cells based on cellular cues including metabolic needs, redox status and varying inter- and intracellular stimuli. The movement of mitochondria is coordinated by a specific set of adapter proteins that connect mitochondria to microtubule motors for transport. The transport of mitochondria to specific subcellular locations influences the local metabolic state and induces cell responses. For example, our work has demonstrated that mitochondrial movement to the leading edge of migrating cells fuels cell migration, a process required for tumor cell metastasis. Projects in the lab focus on determining factors that govern subcellular mitochondrial movement and how local mitochondrial metabolites regulate cell signaling pathways and downstream cell outcomes.