Research
Molecular Mechanisms of Axonal Transport
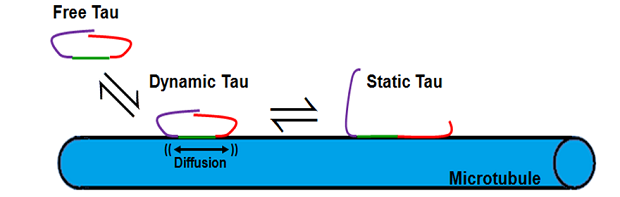
Axonal transport is a critical process in neurons required for the efficient delivery of organelles, proteins, nucleic acids, and small molecules synthesized in the cell body to their site of function in distal regions of the axon. Defects in any one of the protein components in the axonal transport machinery, which includes molecular motor such as kinesin and dynein, the microtubule tracks they move on, a variety of adapter molecules that link motor proteins to their intracellular cargo, and regulatory MAPs (microtubule associated proteins) such as tau, result in serious and often lethal neurodegenerative diseases, including Alzheimer’s, Parkinson’s, Huntington’s, and ALS. Our lab in interested in understanding the interplay between post-translational modifications on both kinesin and tubulin (the protein subunits of microtubules), and different isoforms of tau, in regulating axonal transport under both normal and pathological conditions. We use a combination of molecular biology, fluorescence spectroscopy, and super-resolution single molecule imaging techniques, in both in vitro and in vivo systems, to achieve our goals. Recent results suggest that structural differences in the underlying microtubule lattice dictate tau’s ability to inhibit kinesin-mediated axonal transport in an isoform-specific manner (McVicker et al. (2011) J. Biol. Chem. 286:42873). Current projects are focused on 1.) elucidating the mechanism by which isoforms of tau differentially effect kinesin motility on varied microtubule lattice structures 2.) correlating the in vivo distribution of tau isoforms with different functional effects on both kinesin and microtubules, 3.) understanding the functional consequences of specific point mutations and post-translational modifications in tau associated with known neuronal pathologies, and 4.) examining the role of tau or phosphorylation of the kinesin motor domain in regulating bidirectional transport of intracellular cargo driven by different kinesin family members and cytoplasmic dynein.