Resources
Our laboratories, located in Given E209 and HSRF 309-323, are equipped with state-of-the-art instrumentation which includes: Beckman 640D and NanoDrop 2000 spectrophotometers, Forma ultralow temperature and liquid nitrogen freezers, Beckman refrigerated J26 Avanti XPI high-speed centrifuge, Shandon Cytospin, the Victor X4 multi-label plate reader which reads luminescence, absorbance, fluorescence, Bradford assays, and time-resolved fluorescence assays, analytical and preparative electrophoresis equipment for protein and nucleic acid fractionation, a CHEF-DRII pulse field gel electrophoresis system, thermocyclers, hybridization ovens, an ABI ViiA7 Real-Time PCR apparatus, UV cross-linker, BioRad ChemiDoc gel documentation system, Thermobrite hybridization system for In Situ Hybridization, and bacterial incubators. The laboratory has upright, inverted, and dissecting microscopes with digital imaging capabilities.
Histology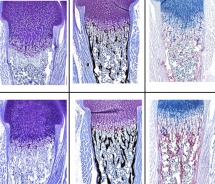
To address the cellular and molecular relationships of bone and cancer, our histology facility is equipped with specialized instrumentation for processing and sectioning tissues, with an emphasis on bone, which includes a Sakura Tissue-Tek Tissue Processor, Sakura Tissue Tek Embedding Station, Hacker-Bright OTF 5000 Cryostat, a Leica RM 2165 paraffin and plastic embedded tissue microtome and for visualization and recording of those sections, we have a Zeiss Axioskop 40 microscope with a AxioCam MRm CCD digital camera and Axiovision imaging software. In addition, we house a Faxitron MX20-DC12 digital radiography system in the UVM mouse quarters.
Tissue culture
 Our research group maintains a dedicated tissue culture facility, operated for the growth and maintenance of approximately 35 cell lines and in an adjacent dedicated space, human embryonic stem (hES) and induced pluripotent (hiPS) cell lines. the laboratory establishes organoid cultures from patient tumors and maintains organoid cell lines from the National Cancer Institute and the Hubracha laboratory. are cultured. The stem cell facility currently maintains two NIH-approved human embryonic stem cell lines, H1 (WA01) and H9 (WA09), obtained from the WiCell Institute of the University of Wisconsin, as well as induced pluripotent (iPS) cell lines. Images are recorded on a Nikon TS-100-F inverted microscope with brightfield and fluorescence options, with a SPOT camera. We have a Leica M 165FC stereo zoom microscope for isolating blastocysts with a Leica DFC 310 FX CCC digital image capture system, dedicated incubators, stem cell processing hoods, and a colony picking hood as well as an oxygenated incubator. Additionally, in an adjacent specialized BSL2 facility, viral vectors are generated, visualized, and recorded using an inverted Nikon TS-100-F microscope with bright field and fluorescence capabilities with SPOT camera and SPOT Advanced software.
Our research group maintains a dedicated tissue culture facility, operated for the growth and maintenance of approximately 35 cell lines and in an adjacent dedicated space, human embryonic stem (hES) and induced pluripotent (hiPS) cell lines. the laboratory establishes organoid cultures from patient tumors and maintains organoid cell lines from the National Cancer Institute and the Hubracha laboratory. are cultured. The stem cell facility currently maintains two NIH-approved human embryonic stem cell lines, H1 (WA01) and H9 (WA09), obtained from the WiCell Institute of the University of Wisconsin, as well as induced pluripotent (iPS) cell lines. Images are recorded on a Nikon TS-100-F inverted microscope with brightfield and fluorescence options, with a SPOT camera. We have a Leica M 165FC stereo zoom microscope for isolating blastocysts with a Leica DFC 310 FX CCC digital image capture system, dedicated incubators, stem cell processing hoods, and a colony picking hood as well as an oxygenated incubator. Additionally, in an adjacent specialized BSL2 facility, viral vectors are generated, visualized, and recorded using an inverted Nikon TS-100-F microscope with bright field and fluorescence capabilities with SPOT camera and SPOT Advanced software.
Imaging
Immunofluorescence microscopy houses two state-of-the-art digital microscopes – an inverted Leica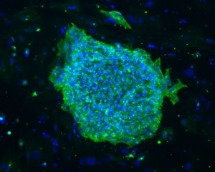 DMI 6000B microscope and an upright Zeiss AxioImager Z2 microscope. Each microscope is equipped with high-resolution CCD cameras, dedicated workstations with access to networked drives, and powerful image analysis software as well as live cell microscopy capabilities. In addition, our group routinely carries out 3D micro-computed tomography for skeletal tissue using a SCANCO 40 medical unit. Multispectral confocal microscopy and CODEX/phenocycler imaging of genes, transcripts, and proteins as well as single-cell genomic analysis, and spatial transcriptomics are routinely utilized.
DMI 6000B microscope and an upright Zeiss AxioImager Z2 microscope. Each microscope is equipped with high-resolution CCD cameras, dedicated workstations with access to networked drives, and powerful image analysis software as well as live cell microscopy capabilities. In addition, our group routinely carries out 3D micro-computed tomography for skeletal tissue using a SCANCO 40 medical unit. Multispectral confocal microscopy and CODEX/phenocycler imaging of genes, transcripts, and proteins as well as single-cell genomic analysis, and spatial transcriptomics are routinely utilized.