Research Interests
My research focuses on the cell biology of viral infections.
Human Immunodeficiency Virus (HIV)
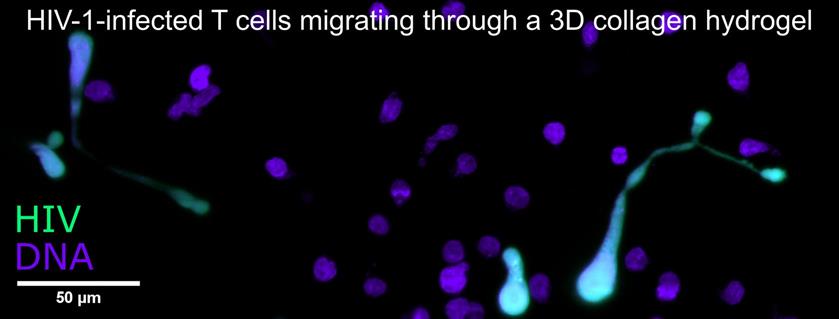
A hallmark of HIV infection is chronic inflammation, possibly due to sensing of HIV-infected T cells and subsequent cytokine production by plasmacytoid dendritic cells (pDCs) and natural killer (NK) cells. Our preliminary work indicates that HIV-induced T cell syncytia (multinucleated cells resulting from cell-cell fusion) may be the primary inducers of cytokine production by pDCs and NK cells. We are currently testing this hypothesis as part of an NIH-funded R21 project in the Thali lab using novel cell isolation tools and functional in vitro pDC/NK cell assays. In parallel, we are studying the motility of syncytia in 3D collagen cultures using long-term light sheet fluorescence microscopy and computational analysis of large 3D cell tracking datasets.
Placental BiologyThe syncytiotrophoblast is the outermost fetal layer of the placenta, which forms due to the fusogenic action of endogenous retroviral glycoproteins, the syncytins. We (the Thali lab) are studying how cell-cell fusion is tightly regulated to ensure proper formation of the syncytiotrophoblast.
Rotavirus (RV)RV is a major cause of diarrheal disease in children and infants in low and middle-income countries, particularly because they do not mount a sufficiently protective immune response to available RV vaccines. In the Lee group, we are investigating this problem by measuring adaptive immune responses to RV vaccination and natural RV infection in infants and children, with my particular focus being on memory B cells.