Anathy lab introduction
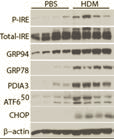
The endoplasmic reticulum (ER) is an active tubular network involved in protein folding, gluconeogenesis, lipid synthesis, mitochondrial fission and calcium storage. Cellular events that demand an increase in protein folding will create an imbalance in protein folding enzymes and will increase unfolded proteins in the ER, eliciting a response called the Unfolded Protein Response (UPR). The UPR initially shuts down selective protein synthesis and activates transcription factors such as ATF6, ATF4 and Xbp-1 that promote synthesis of chaperones (e.g. GRP94 and GRP78), protein disulfide isomerases (PDIs) to correctly fold the misfolded proteins. We have found that the UPR is activated by various agents (allergens, viruses and biological ligands) in the structural and immune cells of the lung.
Currently we are characterizing the functional or pathological role of UPR in:
- allergen-induced lung inflammation and airway fibrotic remodeling,
- influenza virus protein folding and propagation and
- during the differentiation of T helper cells into various subsets (e.g. Th1, Th2 and Th17).
Our laboratory utilizes various systems (primary lung epithelial cultures, human samples and transgenic mouse technology) and techniques (microscopy, molecular (CRISPR-Cas9), biochemical (mass spectrometry) and forced oscillation) to investigate how UPR contributes to the development of inflammation, fibrosis or propagation of influenza virus and ultimately to the pathophysiology of the lung.
Research Projects available:
Currently to understand the functions of UPR-induced protein disulfide isomerases (PDIs) in allergic asthma and influenza infections we have two interesting projects available for seniors/honors undergraduate and graduate students :
1. CRISPR-Cas9 mediated deletion of PDIA 3/5/6 in human lung epithelial cells and characterization of the effect of deletion of these genes in allergen/influenza-induced inflammatory cytokine response.
2. Generation of protein-trap mutants of PDIA 3/5/6 to characterize the interacting client proteins of PDIs during allergen/influenza-infection by mass spectrometry.